運用人工智慧軟體於無精症睪丸檢體轉印抹片之判讀
許程皓1、葉淳甫4、黃奕燊1,2、陳威任1,2、彭昱璟3、蔡承翰1,2、劉庭祿4、杜奕瑾4、黃志賢1,2
1臺北榮民總醫院泌尿部; 2國立陽明交通大學書田泌尿科學研究中心;
3臺北榮民總醫院病理檢驗部; 4台灣人工智慧實驗室
Artificial intelligence interpretation of touch print smear cytology of testicular specimen from patients with azoospermia
Chen-Hao Hsu, M.D., M.T.M1, Chun-Fu Yeh, M.S.4, I-Shen Huang, M.D.1,2, Wei-Jen Chen, M.D. 1,2, Yu-Ching Peng, M.D.3, Cheng-Han Tsai, M.D.1,2, Tyng-Luh Liu, Ph.D. 4, Ethan Tu, M.S. 4,
* William J. Huang, M.D., Ph.D. 1,2 (* Corresponding author)
1 Department of Urology, Taipei Veterans General Hospital, Taipei, Taiwan
2 Department of Urology, School of Medicine, College of Medicine and Shu-Tien Urological
Research Center, National Yang Ming Chiao Tung University, Taipei, Taiwan
3 Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taiwan
4 Taiwan AI Labs4
Purpose: Identification of mature sperm at microdissection testicular sperm extraction (mTESE) is a crucial step of sperm retrieval for the use in the intracytoplasmic sperm injection (ICSI), through which the couples with non-obstructive azoospermia (NOA) can accomplish the fertility task. To enhance the efficiency of sperm identification by the clinician, we have developed a method using touch print smear (TPS) cytology allowing immediate interpretation to provide such information. However, there is a learning curve and technique barrier for reading TPS slides. The application of artificial intelligence (AI) systems using machine learning (ML) and convolutional neural network (CNN) solutions is potentially useful to fill the gap.
Materials and methods: This study retrospectively collected 146 microscopic images of the TPS from the testicular specimen of patients with azoospermia at Taipei Veterans General Hospital, which include the categories of Sertoli cell, primary spermatocytes, round spermatids, elongated spermatids, immature sperm, and mature sperm. Out of the 146 images, 134 images were assigned as the training set and the other 12 were for the validation set. The RetinaNet, a one-stage detection framework, was adopted for the cell detection. The model performance was evaluated at the cell level with average precision (AP) and recall, which were the conventional metrics used for object detection in computer vision.
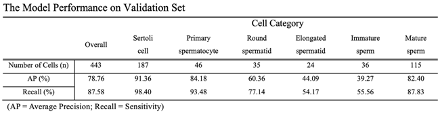
Results: Totally 5408 cells were annotated by an experienced surgeon within the training set, including 2159 Sertoli cells, 369 primary spermatocytes, 423 round spermatids, 279 elongated spermatids, 521 immature sperm, and 1657 mature sperm. The overall AP and recall were 78.76% and 87.58%, respectively. The performance on each category is presented at the table below. This model could detect Sertoli cells, primary spermatocytes and mature sperm better than cells of the other three categories, which is likely attributed to the characteristics shared among these cells.
Conclusion: This study proposed an innovative approach that leveraged ML methods to facilitate the diagnosis of spermatogenesis at mTESE for patients with NOA. It further demonstrated promising results of an AI solution that is capable of detecting and classifying cells from TPS reading. With the assistance of ML techniques, surgeons could determine the stages of spermatogenesis and provide timely histopathological diagnosis for infertile males.